By Paul Gaines, Ph.D. and Christopher Gaines
Used with Permission from Inorganic Ventures - Inorganic Ventures is a leading manufacturer of inorganic standards and custom standards for ICP-OES, ICP-MS, IC and AAS.
The Facts
FACT: Shelf Life does NOT mean expiration date.
FACT: A standard's expiration date should never exceed 1 year.
FACT: A standard's expiration date and shelf life are two entirely different entities.
Chemical stability is only one of many factors involved in defining expiration date and shelf life. This article provides you, the consumer, with the best definitions for shelf life and expiration date. This information will help you make the correct decisions.
The integrity of an aqueous trace metals standard is dependent upon:
- The chemical stability of the standard.
- Transpiration losses of the standard.
- The "human factor" while using the standard.
Shelf Life
The shelf life of aqueous trace metals standards is dependent upon numbers 1 and 2 above. Shelf Life is the amount of time that a properly packaged and stored standard will last without undergoing chemical or physical changes, remaining within the specified uncertainty. A change greater than that uncertainty (±0.5% relative for our standards) means the standard has gone over (passed) its shelf life.
Inorganic Ventures manufactures single-element standards to be chemically stable indefinitely. Our chemists have been checking and testing standards for almost 20 years. Inorganic Ventures can state with certainty that there are no chemical stability problems that have not been solved. Number 1 above has been eliminated in our facility.
All standards have a limited shelf life
A standard's finite shelf life is caused by transpiration (the passage of vapor from within a container to the outside). The entire chemical standard industry suffers from transpiration loss. Inorganic Ventures's scientists have studied these losses over a period of several years. Figure 1 below provides a brief presentation of our transpiration data.
Figure 1 - Transpiration Losses over Time:
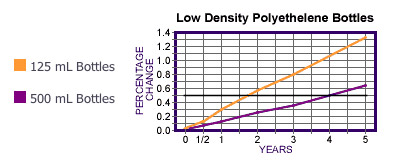
Our studies, performed on our 500 mL and 125 mL LDPE bottles, showed the following:
- Closed but untaped 500 mL bottles have a shelf life of 4 years.
- Closed but untaped 125mL bottles have a shelf life of 21 months.
- Transpiration loss occurs mainly around the cap circumference and not through the container walls.
- There is no difference between the transpiration loss of water versus hydrochloric or nitric acid aqueous solutions.
- The shelf life can be accurately predicted from the ratio of the cap circumference to the surface area of the solution exposed just below the head space.
- Transpiration loss is linear with time.
Typically, Inorganic Ventures stock items have an average shelf life of 2-4 years. Some have a shelf life exceeding a decade.
Inorganic Ventures purchases NIST SRMs that come packaged in 60 mL HDPE bottles. The cap circumference to volume ratio predicts a shelf life of up to one year. NIST has reinforced this fact, stating, "The limit on the validation period is due to transpiration of the solution... A one year shelf life can only be justified." — source
Expiration Dates
A standard's expiration date is dependent upon numbers 1, 2, and 3 above. Inorganic Ventures has eliminated number 1 and greatly reduced number 2. This leaves the "human factor" (number 3). Unfortunately, this is the one element that simply can't be controlled.
Expiration dates should never exceed a year
The expiration date of a standard is defined as the amount of time that it should remain in use after opening. Eventually, human error will contaminate and/or greatly devalue a standard. Most federal and state regulatory agencies recommend expiration dates no longer than one (1) year. Stricter agencies require expiration dates of half that time.
When you use a standard for longer than a year, you are gambling that absolutely nothing has inadvertently affected the chemical components.
Why is the "human factor" so dangerous?
Once a bottle is opened, the "human factor" can cause:
- Contamination of standards from pipet tips, volumetric glassware, and/or switched bottle caps.
- Loosely screwed caps that allow head space to vent.
- Contamination of contents by dust and/or vapors.
- Solution to be poured back into the wrong bottle.
- Contamination by the "wrong" packaging container.
- Solution to be accidentally spilled.
To err is human. It is not intentional, but the law of averages suggests that if something can go wrong, eventually it will.