INTRODUCTION
 Cryogenic samples such as eukaryotic cell-based therapeutics require storage at temperatures below the threshold for the glass transition phase of water (Tg-H2O, approximately -135°C). Storage under those conditions avoids biological activity and minimizes loss of post-thaw cell viability1. Keeping eukaryotic cell solutions at temperatures higher than Tg-H2O introduces serious risks. When encapsulated liver cell spheroids were stored at -80°C, decreased viable cell numbers and cell function were detected after just one month of storage compared with the same cells stored at -170°C2. Liquid nitrogen (LN2) and its vapor phase provide a safe environment for such samples, maintaining temperatures at around -170°C or lower. LN2, first produced in 1883 by Polish physicists, is now used as a coolant in many industrial environments from computers to cameras – and superconductors to vacuum pumps. It is indispensable in any research that involves cryogenic samples. Cryopreservation allows researchers to store biological samples for years without compromising sample integrity.
Cryogenic samples such as eukaryotic cell-based therapeutics require storage at temperatures below the threshold for the glass transition phase of water (Tg-H2O, approximately -135°C). Storage under those conditions avoids biological activity and minimizes loss of post-thaw cell viability1. Keeping eukaryotic cell solutions at temperatures higher than Tg-H2O introduces serious risks. When encapsulated liver cell spheroids were stored at -80°C, decreased viable cell numbers and cell function were detected after just one month of storage compared with the same cells stored at -170°C2. Liquid nitrogen (LN2) and its vapor phase provide a safe environment for such samples, maintaining temperatures at around -170°C or lower. LN2, first produced in 1883 by Polish physicists, is now used as a coolant in many industrial environments from computers to cameras – and superconductors to vacuum pumps. It is indispensable in any research that involves cryogenic samples. Cryopreservation allows researchers to store biological samples for years without compromising sample integrity.
PROBLEM
Biological samples must be collected, processed and stored using best practices to maintain optimal sample quality. It is very difficult to generate robust, reproducible data without high quality samples3. The quality of cryopreserved samples is affected by both storage temperature and sample handling procedures.3,4 An alternative medium often used in biomedical and molecular biology research for temperature management is dry ice, the solid form of carbon dioxide. This material sublimates at -78°C, and its uses are as varied as those for liquid nitrogen. Many laboratories rely on dry ice to maintain a cold environment during transport of their biological materials. However, transporting biosamples on dry ice has some surprising and important drawbacks. Studies show that dry ice results in acidification of sample solutions stored in screwcap tubes and potentially affects protein stability.5 Moreover, post-thaw viability of human lymphocytes suspended in 10 percent DMSO was markedly decreased when they were shipped on dry ice compared to shipment in LN2 vapor-phase6.
Cryopreserved samples must be kept at temperatures lower than -130°C to prevent sample breakdown from enzymatic reactions7. Every temperature increase of 7.8°C doubles the rate of deleterious chemical reactions inside cryopreserved samples8. Temperature excursions above -130°C can lower the quality of cryopreserved samples. Temperature excursions can occur when cryopreserved samples are added to or retrieved from freezers or when samples are shipped. During these times, samples can be exposed to rapid heating and cooling cycles, which can adversely affect cellular integrity and function.
The most surprising result was obtained in tests where 2ml vials of cryogenic samples were transferred from their liquid nitrogen environment into dry ice with their temperatures continuously monitored. The samples reached and surpassed the glass transition temperature of water within 15 to 25 seconds after placement in dry ice9. Astoundingly, this was about two times faster than when these samples were exposed to the ambient environment. How could this be?

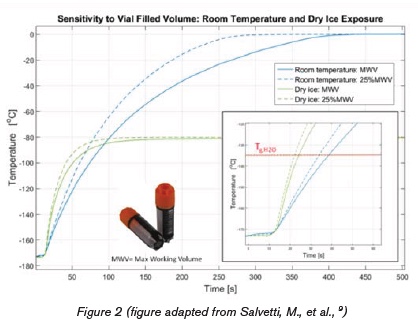
CONVECTION AND CONDUCTION
First, the carbon dioxide sublimation of dry ice results in a microenvironment with enhanced convection – i.e. directed movement of molecules within fluids such as gases. Much like in a convection oven, this effect enables increased heat transmission to any matter placed into this environment. The outcome is an accelerated warm-up of cryogenic samples up to the temperature of the dry ice (-78°C). This warm-up is substantial, as dry ice is about 90°C warmer than the LN2 vapor storage environment of those samples.
Second, the direct contact of the cryogenic tube walls with the dry ice caused an enhanced conductive heat transfer via direct molecular collision to the cryogenic sample tube5. So, researchers who seek to protect their cryogenic material from warming by placing it into dry ice during short transport achieve quite the opposite: a faster warm-up to temperatures above Tg-H2O.
How can such warming events be avoided? Simple. Never placing cryogenic samples into an environment warmer than -135°C. Fortunately, solutions that enable transport of cryogenic samples under these temperatures exist.
CRYOPOD
Brooks Life Sciences offers the cost-effective CryoPod™ Carrier – a portable LN2 vapor-based and lightweight piece of equipment that reliably holds samples at -150°C or colder for over four hours.
- MM Reliability – maintains below -150°C for over 4 hours
- User Safety – LN2 in absorber, under basket, lightweight and ergonomic
- MM Sample safety – no sample contact with LN2 and consistent temperature
With this device, scientists can effectively avoid thermal excursions of their valuable cryogenic samples during handling or transport. This is the reason why it is recommended among best practices for handling cryopreserved cell suspensions4. Check out these CryoPod™ Limited Time Offers.
DOWNLOAD the PDF of this post.
References
1. Hubel A., Spindler R., Skubitz A. Storage of Human Biospecimens: Selection of the Optimal Storage Temperature. Biopreservation and Biobanking. 2014. 12(3): 165-175. DOI: 10.1089/bio.2013.0084. https://www.ncbi.nlm.nih.gov/pubmed/24918763
2. Massie I., Selden C., Hodgson H., Fuller B. Storage temperatures for cold-chain delivery in cell therapy: A study of alginate-encapsulated liver cell spheroids stored at - 80°C or -170°C for up to 1 year. Tissue Engineer Part C: Methods. 2013. 19(3):189–195. doi: 10.1089/ten.tec.2012.0307 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3557435/
3. NCI Best Practices for Biospecimen Resources
4. Simione, F and Sharp, T. Best practices for storing and shipping cryopreserved cells. In Vitro Cell. Dev. Biol. 2017
5. Murphy BM, Swarts S, Mueller BM, van der Geer P, Manning MC, Fitchmun MI. Protein instability following transport or storage on dry ice. Nat Methods. 2013. 10(4):278-9. doi: 10.1038/nmeth.2409. https://www.nature.com/articles/nmeth.2409.pdf
6. Kofanova OA1, Davis K, Glazer B, De Souza Y, Kessler J, Betsou F; ISBER Biospecimen Science Working Group. Viable mononuclear cell stability study for implementation in a proficiency testing program: impact of shipment conditions. Biopreserv Biobank. 2014. 12(3):206-16. doi: 10.1089/bio.2013.0090. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4955601/
7. Mazur, P. Freezing of living cells: mechanisms and implications. Am J Phys. 1984
8. Ogden, S. Temperature, relative humidity, light, and air quality: basic guidelines for preservation. Northeast Document Conservation Center Preservation Leaflets 2.1. 2007
9. Salvetti, M., Fink, J. Barlett, A., Stira, M., Warhurst, J. Thermal excursions of cryogenically frozen vials (below -150°C) and the risk of rising above Tg: analyzing warm-up rates from cryogenic storage to both dry ice and ambient temperature environments. Cytotherapy. 2015. 17(6):S26. https://www.labrepco.com/data/file-downloads/Thermal_excursions_of_cryogenically_frozen_vials__below_-150C__and_the_risk_of_rising_above_Tg_H2O_with_both_dry_ice_and_ambient_environments_1485467833.pdf